INBT's International Research Experience for Students (IRES) program immerses students in bioengineering research projects at IMEC in in Leuven, Belgium. The goal is to cultivate their perceptions of global challenges in STEM, learn how to collaborate with international partners, and develop their research skills.
Tuesday, June 30, 2015
"I can turn a three into a heart!"
It’s difficult to believe that we’ve been here for almost a month already! Now that we’ve really settled into a routine at work, the days simply fly by - suddenly it’s almost 5:30 and you wonder where the day went, lost in culture rooms and in front of microscopes.
This week I started work on a new project, one that focuses on a more efficient way of testing clinical drugs on cardiomyocytes (heart muscle cells). Traditional culture of cardiomyocytes uses big disorganised clusters, which don’t resemble the linear arrangement of cells in an actual heart. Because of this, a significant number of drugs are pulled from the market due to heart issues that weren’t seen in clinical trials. Using a chip designed with alternating lines of hydrophobic (water-resistant) and hydrophilic (‘water-loving’, where cells attach) areas, we’re trying to get the heart cells to grow in single-cell lines to mimic their real-life organisation. There’s plenty of work to be done - because these procedures are still fairly early on, we’re testing all different cell densities, materials to seed the cells on, sterilisation methods, and line designs to see which one gives us the best results!
One of the most comedic moments for me was when I was first introduced to the designs we used that had cells attached. Above each pattern of lines is a number to distinguish which pattern it is, which also becomes hydrophilic during the preparation of the chips. As we prepared the samples for the microscope, my supervisor grinned and said, “Want to see something cool? I can turn a 3 into a heart!” And as I looked into the microscope, there it was indeed - heart cells arranged in a perfect number 3, happily beating away!
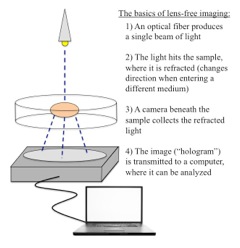
When we go to actually test the cells, we make use of a lens-free imaging setup. The advantage of this over a traditional microscope is that we get a much wider range of view, and it’s much simpler - instead of needing a big nosepiece and all the different lenses, all we need to do is put a laser source above our sample and a camera below, and hook it up to a laptop. (See the image for a bit more description of what goes on!) Once we collect a few seconds of images of the cells beating, we can adjust the images to yield a time-lapse that shows the propagation of the contraction of the cells as they beat, which looks like a wave rolling across the screen. After studying extensively the mechanics of how the signals are conveyed between cells to cause them to contract this year, seeing it in real life with data we’d collected was incredible!
Outside of work, I’ve made it my mission to try every chocolatier and café in Leuven during my time here, and I have to say that I haven’t been disappointed! I’ve taken trips up to London to visit my boyfriend for a weekend and to Bruges, which is an absolutely gorgeous (if very touristy) town. In Bruges, we visited St. John’s Hospital, which has a big museum with the tools of doctors from its time - all I can say is thank goodness medicine has advanced since the 11th century!
Each week here just gets better and better as I get more into my work, and I’m loving the project that I’m working on!
---Becca Black
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment